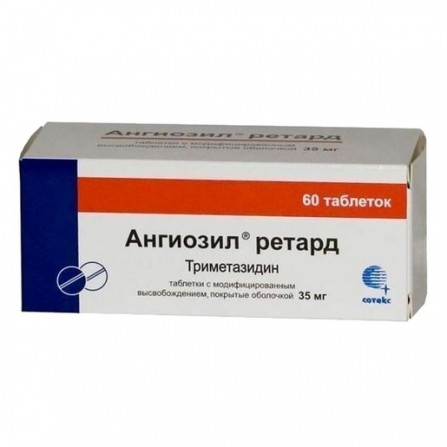
Angiozil retard coated pills 35mg N60
Condition: New product
1000 Items
Rating:
Be the first to write a review!

More info
Active ingredients
Trimetazidine
Release form
Pills
Composition
Trimetazidine dihydrochloride 35 mg; Excipients: microcrystalline cellulose - 0.1206 g, hypromellose - 0.0522 g, colloidal silicon dioxide - 0.0011 g, magnesium stearate - 0.0011 g, opadry II (hypromellose, lactose monohydrate, triacin, macro, a chur; iron oxide red) - 0.008 g
Pharmacological effect
Trimetazidine has an antihypoxic effect. Directly affecting the cardiomyocytes and neurons of the brain, optimizes their metabolism and function. The cytoprotective effect is due to increased energy potential, activation of oxidative decarboxylation and rationalization of oxygen consumption (increased aerobic glycolysis and blockade of fatty acid oxidation). It supports myocardial contractility, prevents the intracellular depletion of adenosine triphosphate and phosphocreatine. Under conditions of acidosis, it normalizes the functioning of membrane ion channels, prevents the accumulation of calcium and sodium in cardiomyocytes, and normalizes the intracellular concentration of potassium ions. Reduces intracellular acidosis and phosphate content due to myocardial ischemia and reperfusion. Interferes with the damaging effects of free radicals, preserves the integrity of cell membranes, prevents activation of neutrophils in the ischemic zone, increases the duration of electrical potential, reduces the release of creatine phosphokinase from cells and the severity of ischemic damage to the myocardium; When angina reduces the frequency of attacks (decreases nitrate consumption); 2 weeks of treatment increases exercise tolerance, decreases blood pressure drops.; Improves hearing and results of vestibular tests in patients with pathology of upper respiratory tract, reduces dizziness and tinnitus. When vascular pathology of the eye restores the functional activity of the retina.
Pharmacokinetics
Absorption and distribution; After taking the drug inside trimetazidine quickly and almost completely absorbed in the gastrointestinal tract. Bioavailability - 90%. The time to reach maximum plasma concentration is 5 hours. Cmax after a single dose of 35 mg of trimetazidine is about 115 ng / ml. Easily penetrates histohematogenous barriers.Binding to plasma proteins - 16%; Elimination; T1 / 2 is about 6.5 hours. Excreted by the kidneys (about 60% - unchanged).
Indications
- IHD: prevention of strokes (in combination therapy); - chorioretinal vascular disorders; - Cochleo-vestibular disorders of the ischemic nature (dizziness, tinnitus, hearing loss).
Contraindications
- Hypersensitivity to any component of the drug; - Lactase deficiency, lactose intolerance, glucose-galactose malabsorption syndrome; - renal failure (CC below 15 ml / min); - severe liver dysfunction; - pregnancy; - breastfeeding period; - age up to 18 years (efficacy and safety have not been established).
Use during pregnancy and lactation
The drug is contraindicated in pregnancy and lactation.
Dosage and administration
Angiosil; retard appoint 1 tab. (35 mg) 2 times / day during the meal in the morning and evening. The duration of therapy is set individually.
Side effects
The frequency of side effects noted when taking trimetazidine is given in the following gradation: very often (more than 1/10); often (more than 1/100. less than 1/10); infrequently (more than 1/1000, less than 1/100); rarely (more than 1/10000, less than 1/1000); very rarely (less than 1/10000, including individual messages); On the part of the digestive system: often - abdominal pain, diarrhea, dyspepsia, nausea, vomiting; ; very rarely - extrapyramidal disorders (tremor, rigidity, akinesia), reversible after discontinuation of the drug.; On the part of the skin: often - skin rash, itching, urticaria; faces.
Overdose
Currently, no cases of overdose have been reported.
Interaction with other drugs
Interaction with other drugs is not described.
special instructions
The drug is not intended for the relief of angina attacks. If an attack of angina occurs, the treatment should be reviewed and adapted. The drug is not indicated for the initial course of treatment of unstable angina or myocardial infarction. Because of the lack of relevant clinical data, the use of the drug is not recommended for patients with severely impaired liver function; retard slightly affects the ability to drive vehicles and perform work that require an increased rate of psychomotor reactions.