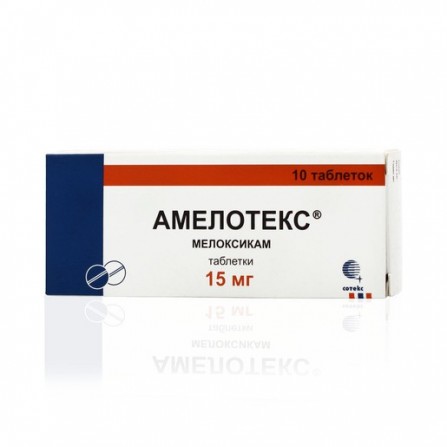
Amelotex pills 15 mg 10 pcs
Condition: New product
1000 Items
Rating:
Be the first to write a review!

More info
Active ingredients
Meloxicam
Release form
Pills
Composition
Solution for intramuscular administration, composition (1 amp. (1.5 ml)): active ingredient: meloxicam - 15 mg; excipients: meglumine; glycofurfural; Poloxamer 188; sodium chloride; glycerol; sodium hydroxide solution 1 M; water for injections
Pharmacological effect
anti-inflammatory, antipyretic, analgesic
Pharmacokinetics
Communication with plasma proteins - 99%. Passes through the histohematic barriers, penetrates into the synovial fluid. Concentration in synovial fluid reaches 50% of Cmax in plasma .; It is derived equally through the intestines and kidneys, mainly in the form of metabolites. Less than 5% of the daily dose is excreted unchanged through the intestine; in the urine, the drug is found in unchanged form only in trace amounts. T1 / 2 meloxicam is 15–20 hours. Plasma clearance averages 8 ml / min. In the elderly, the clearance of the drug is reduced. Vd is low and averages 11 liters. Hepatic or renal failure of moderate severity does not significantly affect the pharmacokinetics of meloxicam.
Indications
rheumatoid arthritis; ; osteoarthritis; ankylosing spondylitis (ankylosing spondylitis); ; inflammatory and degenerative diseases of the joints, accompanied by pain.
Contraindications
hypersensitivity to the active substance or auxiliary components; ; period after coronary artery bypass surgery; uncompensated heart failure; ; anamnestic data on the attack of bronchial obstruction, rhinitis; ; complete or incomplete combination of asthma, recurrent nasal polyposis and paranasal sinuses and intolerance to acetylsalicylic acid and other NSAIDs (including in history); ; erosive and ulcerative changes of the mucous membrane of the stomach or duodenum, active gastrointestinal bleeding; inflammatory bowel disease (ulcerative colitis, Crohn's disease); cerebrovascular or other bleeding; severe liver failure or liver disease in the active phase; severe renal failure in patients not undergoing dialysis (Cl creatinine less than 30 ml / min), progressive kidney disease, incl.confirmed hyperkalemia; ;pregnancy; ; breastfeeding period; ; age up to 15 years .; C caution: to reduce the risk of adverse events, use the minimum effective dose, if possible, with a shorter course, for ischemic heart disease, cerebrovascular diseases, congestive heart failure, dyslipidemia / hyperlipidemia, diabetes mellitus, peripheral artery disease, smoking, Cl of creatinine less than 60 ml / min , history of ulcerative lesions of the gastrointestinal tract, the presence of Helicobacter pylori infection, in old age, with prolonged use of NSAIDs, frequent use of alcohol, severe somatic their diseases, concomitant therapy with the following drugs: anticoagulants (for example, warfarin), antiplatelet agents (for example acetylsalicylic acid, clopidogrel), oral GCS (for example, prednisolone), SIOZS (for example, citalopram, fluoxetine, paroxetine, sertralin).
Dosage and administration
V / m, deep - at 7.5-15 mg / day. With a slight or moderate decrease in renal function (Cl creatinine greater than 25 ml / min), as well as liver cirrhosis in a stable clinical state, dose adjustment is not required. The initial dose in patients with an increased risk of side effects is 7.5 mg / day; The maximum daily dose is 15 mg, and in patients with severe renal insufficiency on hemodialysis, it is 7.5 mg.
Side effects
On the part of the digestive system: nausea, vomiting, belching, abdominal pain, constipation or diarrhea, flatulence, increased activity of hepatic transaminases, hyperbilirubinemia, stomatitis, erosive and ulcerative lesions of the gastrointestinal tract, esophagitis, gastritis, colitis, gastrointestinal perforation, gastrointestinal (hidden or overt), hepatitis .; Nervous system disorders: dizziness, vertigo, headache, tinnitus, confusion, drowsiness, disorientation, emotional lability .; On the part of the respiratory system: bronchospasm .; From the side of blood-forming organs: anemia, leukopenia, thrombocytopenia .; On the part of the cardiovascular system: peripheral edema, increased blood pressure, blood rush to the skin of the face and upper chest, palpitations .; On the part of the urinary system: edema, hypercreatininemia, increasing the concentration of urea in the blood serum; rarely, acute renal failure, interstitial nephritis, albuminuria, hematuria .; From the senses: conjunctivitis, violation (includingblurring) of view .; On the part of the skin: itching, skin rash, urticaria, photosensitivity, bullous rash, erythema multiforme, toxic epidermal necrolysis .; Allergic reactions: angioedema, anaphylactoid, anaphylactic reactions .; Local reactions: possible burning and pain at the injection site.
Overdose
Symptoms: increased side effects .; Treatment: symptomatic. There is no specific antidote and antagonist.
Interaction with other drugs
With simultaneous use with other NSAIDs increases the risk of ulceration of the gastrointestinal tract and gastrointestinal bleeding .; Increases plasma lithium concentration; reduces the effectiveness of intrauterine contraceptives, antihypertensive drugs .; Indirect anticoagulants, ticlopidine, heparin, thrombolytics increase the risk of bleeding; methotrexate enhances mielodepressive effect; diuretics increase the risk of kidney dysfunction; cyclosporine enhances nephrotoxic effect; Kolestiramin accelerates excretion. Myelotoxic drugs increase the hematotoxicity of the drug.
special instructions
In the event of peptic ulcers or gastrointestinal bleeding, the development of side effects from the skin and mucous membranes of the drug should be canceled. In patients with reduced BCC and reduced CF (dehydration, CHF, surgical operations), clinically severe CRF may occur, which is completely reversible after discontinuation of the drug (in such patients, daily diuresis and renal function should be monitored at the beginning of treatment). With a persistent and significant increase in transaminases and changes in other indicators of liver function, the drug should be canceled and control tests should be conducted. In patients with an increased risk of side effects, treatment begins with a dose of 7.5 mg. In the terminal stage of CHF in patients on dialysis, the dose should not exceed 7.5 mg / day; Influence on ability to drive vehicles and work with mechanisms. During the period of treatment, care must be taken when driving vehicles and engaging in other potentially hazardous activities that require increased concentration and psychomotor speed (with the appearance of dizziness and drowsiness).