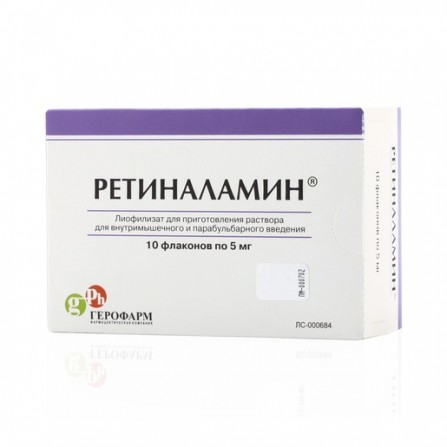
Retinalamin powder lyophilisate for injection 5mg vial N10
Condition: New product
998 Items
Rating:
Be the first to write a review!

More info
Active ingredients
Polypeptide fractions of the retina of livestock
Release form
Lyophilisate
Composition
Each gram of the drug contains: Active substance: clotrimazole 10.0 mg. Excipients: purified talcum 925.0 mg, corn starch 50.0 mg, colloidal silicon dioxide 10.0 mg, cosmetic fragrance 5.0 mg.
Pharmacological effect
Retinalamin is a complex of water-soluble polypeptide fractions with a molecular weight of not more than 10,000 Da. The drug has a stimulating effect on the photoreceptors and cellular elements of the retina, helps to improve the functional interaction of the pigment epithelium and the outer segments of the photoreceptors, glial cells during dystrophic changes, and accelerates the recovery of the light sensitivity of the retina. Normalizes vascular permeability, reduces the manifestations of the local inflammatory reaction, stimulates reparative processes in diseases and injuries of the retina.
Pharmacokinetics
The composition of Retinalamin, the active ingredient of which is a complex of polypeptide fractions, does not allow the usual pharmacokinetic analysis of its individual components.
Indications
Compensated primary open-angle glaucoma, diabetic retinopathy, central dystrophy of inflammatory and traumatic genesis of retina, central retinal dystrophy, myopic disease (as part of complex therapy), central and peripheral tapetoretinal abiotrophy.
Contraindications
Individual hypersensitivity to the components of the drug, up to 18 years of age - with compensated primary open-angle glaucoma, diabetic retinopathy, myopic disease (due to the lack of data on efficacy and safety); age up to 1 year - with central retinal dystrophy of inflammatory and traumatic genesis, central and peripheral tapetoretinal abiotrophy.
Use during pregnancy and lactation
The drug is contraindicated in pregnancy (no data on efficacy and safety). If necessary, the appointment of the drug during lactation breastfeeding should be discontinued.
Dosage and administration
Adults with diabetic retinopathy, central dystrophy of inflammatory and traumatic genesis of retina, central and peripheral tapethoretinal abiotrophy, parabulbarno or intramuscularly, 5-10 mg once a day. The course of treatment is 5 to 10 days; If necessary, repeat after 3 - 6 months. With compensated primary open-angle glaucoma, parabulbarny or intramuscularly, 5 mg once a day. The course of treatment is 10 days; If necessary, repeat after 3-6 months. When myopic disease parabulbarno 5 mg 1 time per day. The course of treatment is 10 days. Children aged 1–5 years with central retinal dystrophy of inflammatory and traumatic genesis, central and peripheral tapethoretinal abiotrophy, parabulbarno or intramuscularly, 2.5 mg 1 time per day. Children aged 6 to 18 years with central dystrophy of inflammation and traumatic genesis of the retina, central and peripheral tapethoretinal abiotrophy parabulbarno or intramuscularly 2.5 to 5.0 mg once a day. The drug is dissolved in 1 - 2 ml of 0.9% sodium chloride solution, directing the needle to the wall of the vial to avoid foaming. The course of treatment is 10 days; If necessary, repeat after 3-6 months.
Side effects
No adverse events reported. There may be allergic reactions in the case of individual hypersensitivity to the components of the drug.
Overdose
No cases of drug overdose have been reported.
Interaction with other drugs
Drug interaction of the drug is not described.
special instructions
Features of the drug at the first admission or with its cancellation are not available. In the case of skipping the injection, it is not recommended to inject a double dose, but to carry out the next injection as usual on the scheduled day. Influence on ability to steer vehicles, mechanisms. Does not affect.