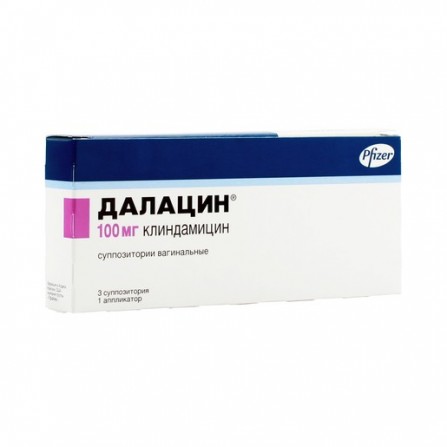
Dalacin vaginal suppositories 100 mg 3 pieces
Condition: New product
999 Items
Rating:
Be the first to write a review!

More info
Active ingredients
Clindamycin
Release form
Suppositories
Composition
1 g: Active substances: clindamycin (in the form of phosphate) - 100 mg. Excipients: solid fat (Witepsol H-32, a mixture of triglycerides, diglycerides and monoglycerides) - about 2.4 g.
Pharmacological effect
Antibiotic group of lincosamides for local use .; Clindamycin phosphate is not active in vitro, but is rapidly hydrolyzed in vivo to form clindamycin with antibacterial activity. Clindamycin inhibits protein synthesis in the microbial cell due to the interaction with the 50S-subunit of ribosomes .; The following microorganisms that cause bacterial vaginosis are sensitive to clindamycin in vitro: Gardnerella vaginalis, Mobiluncus spp., Mycoplasma hominis, Bacteroides spp., Peptostreptococcus spp.
Pharmacokinetics
After clindamycin is administered intravaginally at a dose of 100 mg 1 time / day (in the form of suppositories) for about 3 days, about 30% (6-70%) of the administered dose of clindamycin is absorbed into the systemic circulation, while the AUC is 3.2 mcg / h / ml (0.42-11 mcg / h / ml). Cmax in serum is reached after about 5 hours (1-10 hours) after administration of the vaginal suppository and averages 0.27 mcg / ml (0.03-0.67 mcg / ml) on the 3rd day of therapy .; The systemic effect of clindamycin when administered intravaginally is significantly weaker than when administered in therapeutic doses orally or intravenously; Withdrawal; On average, T1 / 2 is 11 hours (4-35 hours) .; Pharmacokinetics in special groups of patients; Clinical studies of clindamycin in the form of vaginal suppositories involved an insufficient number of patients aged 65 years and older to assess the difference in clinical response to therapy between this age group and younger patients.
Indications
- bacterial vaginosis.
Contraindications
- hypersensitivity to clindamycin, lincomycin or any component of the drug; - antibiotic-associated colitis in history; - age up to 18 years (data on safety and efficacy are not available).
Use during pregnancy and lactation
With the use of clindamycin intravaginally in the II or III trimester of pregnancy, there was no increase in the frequency of congenital abnormalities of the fetus. If dalacin; vaginal suppositories are used during the II or III trimesters of pregnancy (although there have been no official studies on the use of suppositories in pregnant women), an adverse effect on the fetus seems unlikely.The use of the drug in the II-III trimester of pregnancy is possible if the potential benefit to the mother exceeds the risk to the fetus .; Adequate controlled studies on the use of the drug in the first trimester of pregnancy have not been conducted, therefore, Dalacin; vaginal suppositories can be administered to women in the first trimester of pregnancy only by absolute indications, i.e. when the potential benefits of drug therapy outweigh the potential risk to the fetus .; In animal studies with the introduction of clindamycin s / c or inside no negative effects on the fetus were found, except in cases of taking the drug in doses toxic to the mother .; It is not known whether clindamycin is excreted in breast milk after intravaginal use. Clindamycin is found in breast milk after oral or parenteral administration, so during breastfeeding, you should either cancel the use of the drug, or stop breastfeeding, given the degree of importance of the drug to the mother.
Dosage and administration
The recommended dose is 1 intravaginal suppository, preferably at bedtime for 3 consecutive days .; Terms of use of the drug; The introduction of suppositories without an applicator; 1. It is necessary to remove the foil suppository .; 2. Lying on your back, pull your knees up to your chest .; 3. Carefully insert the suppository into the vagina with the help of your middle finger as deep as possible .; The introduction of suppositories using an applicator; 1. The plastic applicator, which is in the package with the drug, is designed to facilitate the introduction of the suppository into the vagina .; 2. It is necessary to remove the foil suppository .; 3. Place the flat end of the suppository in the opening of the applicator .; 4. In the supine position, tighten your knees to your chest .; 5. Holding the applicator horizontally by the ribbed end of the body, gently push it into the vagina as far as possible .; 6. Slowly pressing the plunger, insert the suppository into the vagina .; 7. Carefully remove the applicator from the vagina .; 8. After each use, the applicator should be washed with warm water and soap and let it dry completely.
Side effects
The safety of using clindamycin in the form of suppositories was evaluated in non-pregnant women in clinical studies .; The frequency of adverse reactions is as follows: very often - ≥1 / 10; often - ≥1 / 100, <1/10; infrequently - ≥1 / 1000, <1/100; rarely - ≥1 / 10000, <1/1000; frequency unknown - impossible to estimate based on available data .; Infectious and parasitic diseases: often - fungal infections,caused by fungi of the genus Candida .; On the part of the nervous system: often - headache .; On the part of the digestive tract: often - abdominal pain, diarrhea, nausea; infrequently - vomiting; frequency unknown - pseudomembranous colitis .; On the part of the skin and subcutaneous tissues: often - itchy skin; infrequently - rash .; From the musculoskeletal and connective tissue: infrequently - pain in the side .; On the part of the kidneys and urinary tract: infrequently - pyelonephritis, dysuria .; On the part of the genital organs and the mammary gland: often - vulvovaginal candidiasis, vulvovaginal pain, vulvovaginal disorders; infrequently - vaginal infections, vaginal discharge, menstrual disorders .; Common disorders and reactions at the injection site: infrequently - pain at the injection site, itching at the injection site, localized edema, pain, hyperthermia.
Overdose
With intravaginal use of suppositories Dalacin; absorption of clindamycin in quantities sufficient for the development of systemic reactions is possible .; Accidental ingestion of the drug in the gastrointestinal tract can cause systemic effects similar to those that occur after ingestion of clindamycin in therapeutic doses. Possible systemic side effects include diarrhea, hemorrhagic diarrhea, including pseudomembranous colitis .; Treatment: symptomatic and supportive therapy.
Interaction with other drugs
There is cross-resistance between clindamycin and lincomycin .; In vitro, antagonism between clindamycin and erythromycin has been demonstrated .; Clindamycin has been found to impair neuromuscular transmission and, therefore, may enhance the effect of peripheral muscle relaxants, therefore the drug should be used with caution in patients receiving drugs from this group .; Since there is no information on the use of other drugs for intravaginal administration, the joint use of the drug with other intravaginal drugs is not recommended.
special instructions
Prior to prescribing the drug, vulvovaginitis caused by Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhoeae, Candida albicans and Herpes simplex virus should be excluded using appropriate laboratory methods. Intravaginal use of clindamycin can lead to increased growth of insensitive microorganisms, especially yeast-like fungi .; The use of clindamycin (like almost all antibiotics) orally or parenterally is associated with the development of severe diarrhea and in some cases pseudomembranous colitis.With the development of severe or prolonged diarrhea, the drug should be canceled and, if necessary, carry out appropriate diagnostic and therapeutic measures .; Patients should be warned that during drug therapy, sexual contact should be avoided, as well as the use of other means for intravaginal administration (tampons, douching) .; The drug contains components that can reduce the strength of latex or rubber products (condoms, contraceptive vaginal diaphragms). Therefore, the use of such products during treatment with Dalacin and for 72 hours after application is not recommended .; The use of the drug Dalacin is not recommended; vaginal suppositories during menstruation. The beginning of therapy should be postponed until the end of menstruation .; Influence on the ability to drive vehicles and control mechanisms; The drug does not affect the ability to drive vehicles and engage in potentially hazardous activities that require high concentration of attention and speed of psychomotor reactions.